Biological Control of Sitophilus zeamais in Stored Maize Using Beauveria bassiana
| Received 19 Jun, 2025 |
Accepted 12 Aug, 2025 |
Published 13 Aug, 2025 |
Background and Objective: The increasing demand for sustainable alternatives to chemical pesticides in grain storage has led to growing interest in biological control methods, particularly in developing countries where access to conventional pesticides is limited. This study aimed to evaluate the effectiveness of Beauveria bassiana as a biological control agent against Sitophilus zeamais, a major pest of stored maize. Materials and Methods: Beauveria bassiana was isolated from soil samples collected near oil palm trees in the Ashanti Region of Ghana. Laboratory bioassays were conducted to assess the efficacy of three conidial concentrations (2.0×107 , 3.0×107, and 4.0×107 spores/mL) on adult S. zeamais over 7 Days under controlled conditions (27±2°C, 70±5% RH). Mortality rates were recorded daily, and data were analyzed using appropriate statistical tests to determine significance levels among treatments at p<0.05. Results: A concentration- and time-dependent response was observed. The highest conidial concentration (4.0×107 spores/mL) caused 46% mortality of S. zeamais by Day 7. Statistical analysis confirmed significant differences between treatments during the final phase of the experiment (p = 0.006), demonstrating the increasing effectiveness of B. bassiana with higher dosages and exposure time. Conclusion: The study highlights the potential of B. bassiana as an effective biological control agent against S. zeamais in stored maize. This finding offers a promising alternative to chemical pesticides, especially in resource-limited settings. Further research is recommended to explore its application under field and storage conditions.
| Copyright © 2025 Witsi et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Maize (Zea mays L.) stands as the world’s most extensively produced staple crop, with an estimated annual production of more than 1 billion tonnes1. About 80% of Ghana’s total maize production is from the Eastern, Ashanti, and Brong Ahafo Regions2. As a crucial staple food in Africa, maize constitutes about 38% of Africa’s food supply and provides up to 50% of dietary protein and 80-90% of energy intake in developing countries3,4, making its proper storage essential for year-round food supply and seed preservation.
However, tropical regions in Africa face significant challenges in maize storage, with potential losses of between 20% and 55% due to post-harvest pests such as insects, fungi, and other microorganisms5-7. The maize weevil (Sitophilus zeamais) emerges as one of the most destructive storage pests globally, capable of causing severe grain losses8,9. Hot and humid weather promotes the growth of fungi, particularly Aspergillus flavus, and infestations such as S. zeamais can make this worse by raising the temperature and moisture content of grains, which in turn encourages secondary fungal attacks, producing harmful mycotoxins10,11.
Traditional pest control methods using chemical insecticides have resulted in various problems, including environmental damage, pest resistance, and harmful effects on non-target organisms12,13. Additionally, the high cost of these pesticides makes them increasingly inaccessible to farmers in developing countries. These challenges, combined with concerns over chemical residues in food, have prompted research into alternative control methods, particularly biopesticides12-14.
Beauveria bassiana, an entomopathogenic fungus, has emerged as a promising biological control agent due to its proven effectiveness against various insect pests, including stored grain pests15-18. The fungus operates by infecting hosts through contact or body entry, initiating infection through spore attachment and cuticle penetration using specialized enzymes. The fungus, when inside, then colonizes the insect’s body and releases mycotoxins, leading to death within days19,20.
This study focused on optimizing B. bassiana application conditions and evaluating its efficacy against S. zeamais in stored maize. This approach addresses critical challenges in food security and sustainable agriculture, particularly in Sub-Saharan Africa, where maize weevil infestation significantly impacts food availability and nutritional quality5,6. This is important because reducing post-harvest losses during grain storage strengthens food security in developing countries21.
The exploration of B. bassiana as a biocontrol agent offers several advantages over conventional methods, including reduced negative impact on human health, slower development of pest resistance, minimal environmental impact, and active pest-seeking capability of bioprotectants22. The efficiency of biopesticides can be enhanced through combined application with other biocontrol agents.
This research is particularly relevant for developing countries, where it can help small-scale farmers implement environmentally-friendly pest management practices while reducing reliance on harmful chemicals. The work aims to address the pressing need for effective control of S. zeamais populations in stored maize, particularly given the crop’s significance throughout Sub-Saharan Africa.
MATERIALS AND METHODS
Study design and area: The research employed an experimental design to isolate and evaluate Beauveria bassiana from soil samples for biological control of Sitophilus zeamais in stored maize. The study was conducted in two phases: A field sampling phase for fungal isolation and a laboratory phase for bioassay experiments. Field sampling was carried out in the Ashanti Region of Ghana across three strategic locations selected based on their ecological characteristics and proximity to the research facility from 10-13th June, 2024. These sites included Donyina, located 8.1 km from Kwame Nkrumah University of Science and Technology (KNUST), the KNUST Botanical Gardens, and Kotei, both situated near the KNUST campus perimeter. The sites were chosen to represent diverse soil environments while maintaining practical accessibility for consistent sample handling and processing. The study area experiences a tropical climate characterized by two rainy seasons, with annual rainfall ranging between 1000 and 1500 mm, and average temperatures between 21.5 and 30.7°C, conditions that potentially favour the natural occurrence of entomopathogenic fungi.

|
Sample distribution and collection protocol: Soil samples were distributed non-uniformly across the three sites, with four samples collected from Donyina and two samples each from KNUST Botanical Gardens and Kotei. This uneven distribution was strategically designed to account for Donyina’s larger area and more diverse microhabitats, as previous studies have shown that habitat diversity can significantly influence entomopathogenic fungal distribution23. The higher sampling intensity in Donyina also aligned with historical records of natural pest suppression in this area, suggesting the presence of beneficial soil microorganisms24. The sampling focused on soil around palm trees (Fig. 1), where conditions were hypothesized to be favourable for entomopathogenic fungi25 due to their dense canopy structure, which maintains stable soil moisture levels by reducing direct solar radiation, minimizing evaporation, and creating a humidity-rich microclimate crucial for fungal survival26, along with the continuous addition of organic matter from fallen fronds, which create nutrient-rich conditions, provides stable carbon and nitrogen sources, improves soil structure, and maintains optimal pH levels that support diverse microbial communities27, as well as the presence of various palm-associated insects that serve as natural hosts for entomopathogenic fungi, create continuous cycles of infection and reproduction, and maintain natural reservoirs of fungal inoculum through infected cadavers in the soil ecosystem28. At each sampling point, standardized collection procedures were followed. The surface layer was carefully scraped away using a sterile spatula to minimize contamination from transient surface microorganisms and debris. Samples were then collected from a depth of 10 to 15 cm below the prepared surface, as this depth range has been shown to harbour stable populations of soil-dwelling entomopathogenic fungi29. This specific depth was chosen because it protects from UV radiation and extreme temperature fluctuations while maintaining adequate moisture levels essential for fungal survival30.
Each soil sample (approximately 500 g) was immediately transferred into individual sterile zip lock bags and sealed to prevent cross-contamination and moisture loss. The bags were labelled with masking tape indicating the collection site, date, and sample number, following standard soil microbiological sampling protocols31. All samples were transported to the KNUST microbiology laboratory in insulated containers to minimize temperature fluctuations and stored at 4°C until processing for B. bassiana isolation, as this temperature has been shown to maintain fungal viability while preventing unwanted growth of other microorganisms32.
Isolation and identification of Beauveria bassiana: Laboratory procedures for isolating and identifying B. bassiana from soil samples followed standard microbiological protocols. All glassware and materials were sterilized at appropriate temperatures (160°C for petri dishes, 120°C for test tubes and media) before use. Two primary media were prepared: Peptone water for initial sample processing and Potato Dextrose Agar (PDA) supplemented with chloramphenicol for fungal culture.
The preparation of culture media followed standardized protocols to ensure reproducibility. Peptone water was prepared by dissolving 10 g of peptone and 5 g NaCl per liter of distilled water, with pH adjusted to 7.2±0.2. The PDA was prepared using 200 g peeled and diced potato infusion, 20 g dextrose, and 15 g agar per liter of distilled water, with final pH adjusted to 5.6±0.2. Both media were autoclaved at 121°C for 15 min at 15 psi. Chloramphenicol (0.05 g/L) was added to PDA after cooling to approximately 45°C to prevent bacterial contamination. For long-term storage, pure cultures of B. bassiana were maintained on PDA slants at 4°C and sub-cultured every three months for six months. Additionally, a glycerol stock was prepared by suspending conidia in 15% sterile glycerol solution and stored at -80°C for culture preservation. Working cultures were maintained on PDA plates at 25°C and sub-cultured every 14 Days to ensure viability and consistent morphological characteristics. All media preparation and culture handling were performed under aseptic conditions in a laminar flow hood to prevent contamination. Serial dilutions were prepared from each soil sample using the standard ten-fold dilution method to 10-3. The 1 mL from each dilution was plated on PDA and incubated at 30°C for 7 Days. Following incubation, colonies were characterized based on morphological features.
Bioassay of Beauveria bassiana against Sitophilus zeamais: The bioassay experiment was designed to evaluate the efficacy of B. bassiana against S. zeamais under controlled laboratory conditions. The methodology encompassed insect rearing, fungal preparation, and mortality assessment protocols33,34.
Adult S. zeamais specimens were obtained through controlled infestation of sterilized maize. The 5 kg of maize were placed in a sterile transparent container covered with mesh netting and maintained in the Faculty of Agriculture Insectary under optimal conditions (27±2°C, 70±5% relative humidity) for natural infestation. After 3-4 weeks of storage, adult weevils were collected through sieving and manual selection.
The selected conidial concentrations (2.0×107, 3.0×107, and 4.0×107 spores/mL) were chosen based on previous efficacy studies of entomopathogenic fungi against stored product pests35. These concentrations fall within the range shown to be effective against various Coleoptera species while remaining economically feasible for practical application36. Preliminary trials in our laboratory also indicated that concentrations below 2.0×107 spores/mL showed minimal efficacy, while those above 4.0×107 spores/mL did not yield significantly higher mortality rates to justify their increased production costs.
The experimental design incorporated several measures to control environmental variables and minimize bias. Treatment groups were arranged in a completely randomized design on laboratory shelves, with positions rotated daily to account for potential microclimatic variations. Environmental conditions were monitored using calibrated data loggers (HOBO U23 Pro v2, Onset Computer Corporation, USA) placed at multiple points within the experimental area. Temperature was maintained at 27±2°C using thermostat-controlled heating/cooling systems, while relative humidity (70±5%) was regulated using a humidifier and monitored with digital hygrometers. Light conditions were standardized to a 12:12-hrs light: Dark cycle using automated fluorescent lighting. To minimize external contaminations, all petri dishes were sealed with parafilm and handled using sterile techniques. Control treatments were physically separated from fungal treatments to prevent cross-contamination while maintaining identical environmental conditions.
For the bioassay, conidial suspensions of B. bassiana were prepared at three concentrations: 2.0×107 (C1), 3.0×107 (C2), and 4.0×107 (C3) spores/mL. The concentrations were achieved by harvesting conidia from pure cultures using a sterile brush and suspending them in sterile distilled water. Initial spore concentration was determined using a haemocytometer and adjusted through serial dilution to achieve the target concentrations.
The experimental setup consisted of five replicates per concentration, with each replicate comprising a sterile petri dish containing filter paper treated with 1 mL of the respective conidial suspension. Control treatments received 1 mL of sterile distilled water. After the treated papers had dried, ten adult weevils were introduced into each petri dish and covered. Mortality was monitored at 24 hrs intervals for 7 Days (168 hrs). Dead insects were removed daily to prevent secondary colonization. The progressive mortality pattern observed across all treatments is shown in Fig. 1.
To confirm fungal involvement, a re-isolation technique was employed where dead weevils were incubated on water-saturated tissue paper within a controlled environment, allowing potential fungal growth to manifest externally37. This method critically distinguished between direct and incidental mortality. A blank formulation control using water demonstrated the specificity of B. bassiana’s entomopathogenic action, as weevils exposed to the control treatment showed no mortality, thereby eliminating alternative explanations for weevil mortality38. These multiple lines of evidence morphological changes, re-isolation technique, and controlled comparison provided a strong confirmation of B. bassiana’s direct pathogenic impact on the target insect population.
Data analysis: Mortality data were analysed using One-way Analysis of Variance (ANOVA) to determine significant differences between treatment concentrations at each time point. The mean number of dead weevils per treatment was calculated daily for each concentration. Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY). Results are presented as Means±Standard Deviation, with different superscript letters indicating significant differences between treatments. A post hoc multiple comparisons were conducted using Tukey’s Honestly Significant Difference (HSD) test to separate treatment means when ANOVA indicated significant differences (p<0.05). Treatments sharing the same letter are not significantly different from each other.
RESULTS
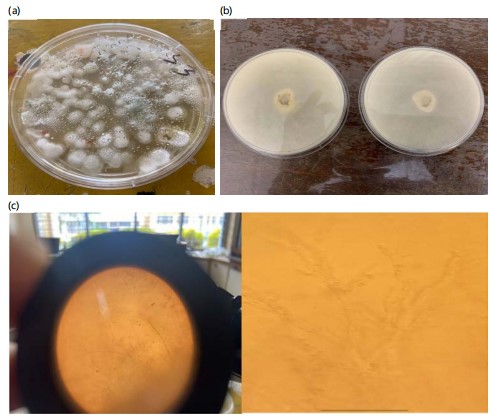
Temporal effects of B. bassiana concentration on S. zeamais mortality: The pathogenicity of B. bassiana against S. zeamais varied with both concentration and exposure time. All three concentrations demonstrated a progressive increase in weevil mortality over the 7 Day observation period (Table 1). Initially, no mortality was observed across all treatments during the first 24 hrs. By Day 7, the highest concentration (C3 = 4.0×107 spores/mL) achieved maximum effectiveness with an average mortality of 4.6 weevils, while the medium concentration (C2 = 3.0×107 spores/mL) resulted in 3.4 weevil deaths, and the lowest concentration (C1 = 2.0×107 spores/mL) caused 2.0 weevil deaths. The pathogenicity of B. bassiana against Sitophilus zeamais was assessed through multiple diagnostic indicators. The Beauveria bassiana colonies exhibited distinctive characteristics: Cottony white appearance on the surface, pale yellow reverse colouration, and well-defined circular edges (Fig. 2a). Pure cultures were obtained through the streak plate technique on PDA and incubated under the same conditions (Fig. 2b).
Morphological identification was confirmed through microscopic examination at 400× magnification (Fig. 2c). The fungus displayed characteristic features, including branched hyphae with hyaline, round to oval single cells. These morphological characteristics aligned with standard descriptions of B. bassiana, confirming successful isolation of the target organism.
Effects of spore concentration on weevil mortality: The comparative analysis of concentration effects revealed statistically significant differences in mortality rates over time (Table 1). During the initial phase (Days 0-3), mortality differences between concentrations were minimal and statistically non-significant (f = 0.75, p = 0.493). A transition period was observed during Days 4-5, where mortality increased significantly (Table 1).
|
Significant treatment effects emerged during the final experimental phase (Days 6-7). Day 6 showed marked differences between concentrations (p = 0.006), with C3 causing the highest mortality (3.8±0.837), followed by C2 (2.8±0.837) and C1 (1.4±1.140). This pattern intensified by Day 7 (p = 0.006), with the highest concentration recording a mean mortality of 4.6±0.894.
DISCUSSION
The study presents compelling evidence for the efficacy of Beauveria bassiana as a biocontrol agent against Sitophilus zeamais, demonstrating both concentration-dependent and time-dependent effects in controlled laboratory conditions. The results merit detailed examination in the context of existing literature and practical applications.
The concentration effects reveal a clear pattern of increasing efficacy with higher spore concentrations. The highest concentration tested (C3 = 4.0×107 spores/mL) achieved the maximum mortality rate of 4.6 weevils by Day 7, while lower concentrations showed proportionally reduced effectiveness. This dose-dependent relationship aligns with previous research demonstrating that increased spore density enhances pathogen impact by improving the likelihood of spore contact and subsequent infection39,40. The observed pattern of effectiveness correlates with broader studies on B. bassiana’s capacity to control stored product pests, supporting its potential as a biological control agent41,42.
The temporal progression of mortality provides crucial insights into the biocontrol mechanism. The absence of mortality during the first 24 hrs across all treatments, followed by gradually increasing mortality rates, reflects the systematic nature of the infection process. This pattern is consistent with the established understanding of B. bassiana’s infection mechanism, which involves initial spore attachment, cuticle penetration, and subsequent host colonization19,20,43. The progressive increase in mortality rates aligns with the documented multi-step process of fungal infection leading to host death, as observed in previous studies44.
Infected maize weevil cadavers exhibited characteristic red colouration and rigid body posture, which are well-documented signs of fungal infection in entomopathogenic systems45. The characteristic red coloration and rigid posture observed in infected weevil cadavers provide strong morphological evidence of successful B. bassiana colonization, consistent with other findings38 on fungal-induced post-mortem changes in insect hosts. The successful re-isolation of the fungus from infected cadavers through controlled incubation confirms Koch’s postulates and validates B. bassiana as the primary mortality agent. This is particularly significant given that control treatments showed no mortality, eliminating alternative explanations for weevil death. These morphological indicators, combined with the re-isolation results, support previous research demonstrating that successful B. bassiana infection produces distinctive post-mortem characteristics in infected insects. The controlled humidity conditions during re-isolation provided optimal conditions for external fungal growth, allowing for definitive confirmation of infection, a methodology that aligns with standard protocols for confirming entomopathogenic fungal activity38.
Statistical analysis of the results revealed a clear progression in treatment efficacy over time. The initial phase (Days 0-3) showed non-significant differences between treatments (f = 0.75, p = 0.493), reflecting the lag time required for infection establishment. The transition period during Days 4-5 marked the onset of observable effects, with mortality differences approaching statistical significance. The final phase of infection demonstrated highly significant differences between treatments (p = 0.006), confirming the cumulative nature of the biological control effects. This pattern strongly supports recent research on the importance of environmental competence in microbial pest control applications46.
The study’s controlled environmental conditions (27±2°C, 70±5% relative humidity) played a crucial role in treatment efficacy. These parameters align with optimal conditions for B. bassiana pathogenicity identified in previous research47. The importance of maintaining appropriate environmental conditions for maximizing fungal efficacy has been well-documented in recent studies, highlighting the critical role of temperature and humidity in successful biological control applications48.
The practical implications of these findings extend beyond immediate pest control efficacy. The results present B. bassiana as a viable alternative to chemical pesticides, addressing longstanding concerns about environmental damage and pest resistance development associated with chemical control methods15,16,18,49. This biological approach aligns with broader goals of strengthening food security through reduced post-harvest losses, particularly relevant in developing countries50.
When considering comparative efficacy, the highest concentration, achieving 46% mortality by Day 7, warrants careful analysis. While this mortality rate may be lower than some chemical treatments, it offers significant advantages in terms of environmental sustainability and resistance management. These benefits include reduced environmental impact, lower risk of pest resistance development, and enhanced compatibility with integrated pest management approaches51.
The findings are of particular significance in the context of post-harvest losses in tropical regions, where insects and fungi can cause up to 40% reduction in stored grain quantity and quality52. The progressive nature of B. bassiana’s effect, though slower than chemical alternatives, provides sustainable long-term control potential, supporting previous observations about the role of fungal entomopathogens in sustainable agriculture.
The results suggest potential optimization strategies for practical applications, including consideration of higher concentrations, extended exposure periods, and integration with complementary control methods. This multi-faceted approach aligns with recent assessments of filamentous fungi’s potential in biological control52. The effectiveness of such integrated approaches has been demonstrated in previous studies, suggesting enhanced pest control efficiency through the combined application of multiple biocontrol agents51.
These findings make a significant contribution to the growing evidence base supporting biological control as a sustainable alternative to chemical pesticides in stored grain protection. The results are particularly relevant for developing countries, where the high cost of chemical pesticides often presents a significant barrier to effective pest management.
CONCLUSION
This study demonstrates that Beauveria bassiana exhibits significant potential as a biological control agent against Sitophilus zeamais in stored maize. The research confirms both concentration-dependent and time-dependent effects, with higher concentrations achieving higher mortalities. While the observed maximum mortality rate of 46% at the highest spore concentration may be lower than chemical alternatives, B. bassiana offers substantial advantages in terms of environmental sustainability and resistance management. The progressive nature of the fungal infection, evidenced by the temporal pattern of mortality, suggests that optimizing application conditions and concentration levels could enhance spore efficacy. These findings support the development of sustainable pest management strategies, particularly valuable for developing countries seeking cost-effective alternatives to chemical pesticides. Future research should focus on investigating higher concentrations, extended exposure periods, and potential synergistic effects with other control methods to maximize the practical application of B. bassiana in stored grain protection.
SIGNIFICANCE STATEMENT
This study identified Beauveria bassiana as an effective biocontrol agent against Sitophilus zeamais, which could be beneficial for sustainable pest management in stored maize. The findings highlight reduced dependency on chemical pesticides, improved post-harvest grain preservation, and enhanced food security. This study will assist researchers in uncovering critical areas of eco-friendly pest control strategies that have remained unexplored by many. Consequently, a new theory on fungal-based biopesticide integration into grain storage systems may be developed.
ACKNOWLEDGMENT
We are thankful to Mr. Eric Acheampong and Mr. Malik Mohammed, both laboratory technologists whose technical expertise and assistance was very vital to the completion of this study. Our gratitude also goes to the Department of Theoretical and Applied Biology, the Faculty of Agriculture and the Department of Pathology all of the Kwame Nkrumah University of Science and Technology, Ghana, for providing us laboratory space to conduct this study.
REFERENCES
- Acheampong, M.A., C.A. Coombes, S.D. Moore and M.P. Hill, 2020. Temperature tolerance and humidity requirements of select entomopathogenic fungal isolates for future use in citrus IPM programmes. J. Invertebr. Pathol., 174.
- Adefunke, J.A.Y. and J.M. Elizabeth, 2018. Influence of using different drying technique on the level of toxigenic Aspergillus flavus in maize (Zea mays) seeds. J. Adv. Microbiol., 11.
- Agrawal, P.K., M.G. Mallikarjuna and H.S. Gupta, 2018. Genetics and applied genomics of quality protein maize for food and nutritional security. Biotechnologies Crop Improv., 3: 151-178.
- Baek, S., M.Y. Noh, S. Mun, S.J. Lee, Y. Arakane and J.S. Kim., 2022. Ultrastructural analysis of beetle larva cuticles during infection with the entomopathogenic fungus, Beauveria bassiana. Pest Manage. Sci., 78: 3356-3364.
- Baki, D., H.S. Tosun and F. Erler, 2021. Efficacy of indigenous isolates of Beauveria bassiana (Balsamo) Vuillemin (Deuteromycota: Hyphomycetes) against the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Egypt. J. Biol. Pest Control, 31.
- Bamisile, B.S., C.K. Dash, K.S. Akutse, R. Keppanan and L. Wang, 2018. Fungal endophytes: Beyond herbivore management. Front. Microbiol., 9.
- Baron, N.C., E.C. Rigobelo and D.C. Zied, 2019. Filamentous fungi in biological control: Current status and future perspectives. Chil. J. Agric. Res., 79: 307-315.
- Barra, P., L. Rosso, A. Nesci and M. Etcheverry, 2013. Isolation and identification of entomopathogenic fungi and their evaluation against Tribolium confusum, Sitophilus zeamais and Rhyzopertha dominica in stored maize. J. Pest Sci., 86: 217-226.
- Belagalla, N., R. Kaur, G.J. Abhishek, 2024. Eco-friendly and targeted through next-generation approaches to insect pest management. Uttar Pradesh J. Zool., 45: 73-99.
- Boullouz, M., P.S. Bindraban, I.N. Kissiedu, A.K.K. Kouame, K.P. Devkota and W.K. Atakora, 2022. An integrative approach based on crop modeling and geospatial and statistical analysis to quantify and explain the maize (Zea mays) yield gap in Ghana. Front. Soil Sci., 2.
- Clay, P.A., M.H. Cortez and M.A. Duffy, 2021. Dose relationships can exacerbate, mute, or reverse the impact of heterospecific host density on infection prevalence. Ecology, 102.
- Devi, G., 2019. Interaction between entomopathogenic nematodes and entomopathogenic fungi in biocontrol mechanism. J. Entomol. Zool. Stud., 7: 959-964.
- Tola, D.M. 2022. Efficacy of indigenous knowledge and selected modified storage structures to insect pests of maize during home storage: A review. Food Sci. Nutr. Technol., 7.
- Wahjono, T.E., Y. Yuliani and Hadiyanto, 2024. Beauveria bassiana; insect pathogen and biopesticide producer as an effective and environmentally friendly alternative for biological control. J. Ilmiah Agrineca, 24: 97-112.
- Asibe, F.A., P.M. Ngegba, E. Mugehu and C.G Afolabi, 2023. Status and management strategies of major insect pests and fungal diseases of maize in Africa: A review Afr. J. Agric. Res., 19: 686-697.
- Gilbert, M.K., A. Medina, B.M. Mack, M.D. Lebar and A. Rodríguez et al., 2018. Carbon dioxide mediates the response to temperature and water activity levels in Aspergillus flavus during infection of maize kernels. Toxins, 10.
- Hintènou, M.V., A.A. Omoloye, O.K.D. Kpindou, M.F. Karlsson, R. Djouaka, A.H. Bokonon-Ganta and M. Tamȯ, 2023. Pathogenicity of Beauveria bassiana (Balsamo-Crivelli) and Metarhizium anisopliae (Metschnikoff) isolates against life stages of Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Egypt. J. Biol. Pest Control, 33.
- Inglis, G.D., J. Enkerli and M.S. Goettel, 2012. Laboratory Techniques Used for Entomopathogenic Fungi. In: Manual of Techniques in Invertebrate Pathology, Lacey, L.A. (Ed.), Elsevier, Amsterdam, Netherlands, ISBN: 978-0-12-386899-2 pp: 189-253.
- Jaber, S., A. Mercier, K. Knio, S. Brun and Z. Kambris et al., 2016. Isolation of fungi from dead arthropods and identification of a new mosquito natural pathogen. Parasites Vectors, 9.
- Jaronski, S.T., 2010. Ecological factors in the inundative use of fungal entomopathogens. BioControl, 55: 159-185.
- Kamarudin, M.A., S. Abdullah and W.H. Lau, 2022. Efficacy of soil-borne entomopathogenic fungi against subterranean termite, Coptotermes curvignathus Holmgren (Isoptera: Rhinotermitidae). Egypt. J. Biol. Pest Control, 32.
- Kaur, R., D. Choudhary, S. Bali, S.S. Bandral and V. Singh et al., 2024. Pesticides: An alarming detrimental to health and environment. Sci. Total Environ., 915.
- Klingen, I. and S. Haukeland, 2006. The Soil as a Reservoir for Natural Enemies of Pest Insects and Mites with Emphasis on Fungi and Nematodes. In: An Ecological and Societal Approach to Biological Control, Eilenberg, J. and H.M.T. Hokkanen (Eds.). Springer, Netherlands, pp: 145-211.
- Kumar, D. and P. Kalita, 2017. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods, 6.
- Kumar, S., S. Kumar, D. Bhandari and M.P. Gautam, 2020. Entomopathogens, pathological symptoms and their role in present scenario of agriculture: A review. Int. J. Curr. Microbiol. Appl. Sci., 9: 2110-2124.
- Lomer, C.J., R.P. Bateman, D.L. Johnson, J. Langewald and M. Thomas, 2001. Biological control of locusts and grasshoppers. Annu. Rev. Entomol., 46: 667-702.
- López-Castillo, L.M., S.E. Silva-Fernández, R. Winkler, D.J. Bergvinson, J.T. Arnason and S. García-Lara, 2018. Postharvest insect resistance in maize. J. Stored Prod. Res., 77: 66-76.
- Mascarin, G.M. and S.T. Jaronski, 2016. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol., 32.
- Meyling, N.V. and J. Eilenberg, 2007. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control, 43: 145-155.
- Meyling, N.V., K. Thorup-Kristensen and J. Eilenberg, 2011. Below- and aboveground abundance and distribution of fungal entomopathogens in experimental conventional and organic cropping systems. Biol. Control, 59: 180-186.
- Mishra, S., P. Kumar and A. Malik, 2015. Effect of temperature and humidity on pathogenicity of native Beauveria bassiana isolate against Musca domestica L. J. Parasitic Dis., 39: 697-704.
- Mondal, S. and P.K. Singh, 2022. In silico host-pathogen surface interaction of entomopathogeic fungi, Beauveria bassiana. Cambridge Open Engage.
- Oliveira, I., J.A. Pereira, E. Quesada-Moraga, T. Lino-Neto, A. Bento and P. Baptista, 2013. Effect of soil tillage on natural occurrence of fungal entomopathogens associated to Prays oleae Bern. Sci. Hortic., 159: 190-196.
- Oyetunde, O.A., G.A.S. Benson, B.T. Osho, A.K. Oyetunde and G.A. Adeshina, 2024. Estimation of genetic variability in maize genotypes under infestation by the maize storage weevil, Sitophilus zeamais using multivariate analysis. Int. J. Plant Soil Sci., 36: 241-250.
- Pawar, V.D., S.B. Bramhankar, R.W. Ingle, R.M. Wadaskar and D.G. Tathod, 2024. Beauveria bassiana: A promising biocontrol agent for Helicoverpa armigera. Int. J. Adv. Biochem. Res., 8: 96-101.
- Pedrini, N., É.K.K. Fernandes and I.M. Dubovskiy, 2024. Multifaceted Beauveria bassiana and other insect-related fungi. J. Fungi, 10.
- Peschiutta, M.L., V.D. Brito, C.R. Krapacher, F. Achimón, R.P. Pizzolitto, D.M.S. Juliana and M.P. Zunino, 2024. Bioinsecticide textile bags for control of maize weevil during grain storage. Crop Prot., 180.
- Prasanna, B.M., N. Palacios-Rojas, F. Hossain, V. Muthusamy and A. Menkir et al., 2020. Molecular breeding for nutritionally enriched maize: Status and prospects. Front. Genet., 10.
- Quesada-Moraga, E., N. González-Mas, M. Yousef-Yousef, I. Garrido-Jurado and M. Fernández-Bravo, 2024. Key role of environmental competence in successful use of entomopathogenic fungi in microbial pest control. J. Pest Sci., 97: 1-15.
- Quesada-Moraga, E., J.A. Navas-Cortés, E.A.A. Maranhao, A. Ortiz-Urquiza and C. Santiago-Álvarez, 2007. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res., 111: 947-966.
- Humber, R.A., 2012. Identification of Entomopathogenic Fungi. In: Manual of Techniques in Invertebrate Pathology, Lacey, L.A. (Ed.), Elsevier, Amsterdam, Netherlands, ISBN: 978-0-12-386899-2, pp: 151-187.
- Ricker-Gilbert, J., O. Omotilewa and D. Kadjo, 2022. The economics of postharvest loss and loss-preventing technologies in developing countries. Annu. Rev. Resour. Econ., 14: 243-265.
- Tariq, S., 2024. Unveiling the effectiveness of Beauveria bassiana as a biocontrol agent against adult hoppers: A review. Zeugma Biol. Sci., 5: 1-9.
- Taylan, Z.Ş. and M.K. Er, 2024. Pathogenicity of Beauveria bassiana (Balsamo) Vuillemin F7-1 on Sitophilus oryzae L. (Coleoptera: Curculionidae) adults and the effect of ambient humidity. J. Agric., 7: 10-16.
- Serna-Saldivar, S.O., 2023. Maize. In: ICC Handbook of 21st Century Cereal Science and Technology, Shewry, P.R., H. Koksel and J.R.N. Taylor (Eds.), Elsevier, Amsterdam, Netherlands, ISBN: 978-0-323-95295-8, pp: 131-143.
- Chowdhury, S.K., M. Banerjee, D. Basnett and T. Mazumdar, 2024. Natural pesticides for pest control in agricultural crops: An alternative and eco-friendly method. Plant Sci. Today, 11: 433-450.
- Swathy, K., M.K. Parmar and P. Vivekanandhan, 2024. Biocontrol efficacy of entomopathogenic fungi Beauveria bassiana conidia against agricultural insect pests. Environ. Qual. Manage., 34.
- Aydin, N.T., E. Tozlu and G. Tozlu, 2023. Potential for the use of the entomopathogenic fungus Beauveria bassiana in the biological control of Cryptolestes ferrugineus and Acanthoscelides obtectus. Turk. J. Biodivers., 6: 88-96.
- Trinh, D.N., T.K.L. Ha and D. Qiu, 2020. Biocontrol potential of some entomopathogenic fungal strains against bean aphid Megoura japonica (Matsumura). Agriculture, 10.
- Temesgen, T. and E. Getu, 2023. Comparing maize storage technologies for managing post-harvest loss in Bako, Ethiopia. Int. J. Agric. Sci. Food Technol., 9: 054-058.
- Vega, F.E., N.V. Meyling, J.J. Luangsa-ard and M. Blackwell, 2012. Fungal Entomopathogens. In: Insect Pathology, Vega, F.E. and H.K. Kaya (Eds.), Elsevier, Amsterdam, Netherlands, ISBN: 978-0-12-384984-7, pp: 171-220.
- Watson, D.W., C.G. Geden, S.J. Long and D.A. Rutz, 1995. Efficacy of Beauveria bassiana for controlling the house fly and stable fly (Diptera: Musciadae). Biol. Control, 5: 405-411.
How to Cite this paper?
APA-7 Style
Witsi,
E., Yeboah,
O.K., Eshun,
B.A., Nyarko,
D.O., Mac-Arthur,
C.K., Amemo,
R.E., Osisiogu,
E.U., Baidoo,
P.K. (2025). Biological Control of Sitophilus zeamais in Stored Maize Using Beauveria bassiana. Science International, 13(1), 70-80. https://doi.org/10.17311/sciintl.2025.70.80
ACS Style
Witsi,
E.; Yeboah,
O.K.; Eshun,
B.A.; Nyarko,
D.O.; Mac-Arthur,
C.K.; Amemo,
R.E.; Osisiogu,
E.U.; Baidoo,
P.K. Biological Control of Sitophilus zeamais in Stored Maize Using Beauveria bassiana. Sci. Int 2025, 13, 70-80. https://doi.org/10.17311/sciintl.2025.70.80
AMA Style
Witsi
E, Yeboah
OK, Eshun
BA, Nyarko
DO, Mac-Arthur
CK, Amemo
RE, Osisiogu
EU, Baidoo
PK. Biological Control of Sitophilus zeamais in Stored Maize Using Beauveria bassiana. Science International. 2025; 13(1): 70-80. https://doi.org/10.17311/sciintl.2025.70.80
Chicago/Turabian Style
Witsi, Esther, Obed Kyei Yeboah, Barbara A. Eshun, Davina O. Nyarko, Cyril K. Mac-Arthur, Raphael E. Amemo, Emmanuel U. Osisiogu, and Philip K. Baidoo.
2025. "Biological Control of Sitophilus zeamais in Stored Maize Using Beauveria bassiana" Science International 13, no. 1: 70-80. https://doi.org/10.17311/sciintl.2025.70.80

This work is licensed under a Creative Commons Attribution 4.0 International License.



