Prevalence of Colistin-Resistant Bacteria in Water Sources in Wa, Upper West Region of Ghana
| Received 18 Aug, 2024 |
Accepted 19 Nov, 2024 |
Published 20 Nov, 2024 |
Background and Objective: Antimicrobial resistance is a major global public health threat, with the highest mortality rate in Sub-Saharan Africa. Colistin is considered the last line of defense against multidrug-resistant Gram-negative pathogens. This study sought to assess the prevalence of colistin-resistant bacteria in water sources in the Wa Municipality of the Upper West Region of Ghana.Materials and Methods: A cross-sectional study was conducted, collecting 40 water samples from boreholes, wells and taps in four communities within the Wa Municipality. Samples were cultured and isolates were identified using standard biochemical tests. Colistin susceptibility was assessed using Kirby-Bauer disk diffusion and microbroth dilution techniques, with results interpreted using EUCAST standards. Data was analysed using SPSS version 28. Results: Diverse Gram-negative enteric organisms were isolated, including Citrobacter species (28.5%), Klebsiella species (25%), Serratia marcescens (21.4%), and others. Microbroth dilution testing revealed 44.8% of isolates were colistin-resistant, compared to only 4.7% by disk diffusion. Resistant organisms were found across all communities and water sources. Urban areas showed a significant association with colistin resistance (p = 0.049). Conclusion: High colistin resistance in Wa water sources indicates sanitation challenges. Improved water treatment and antibiotic stewardship are crucial. Ongoing surveillance is recommended.
| Copyright © 2024 Osisiogu et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
The introduction of antibiotics into medicine is arguably the greatest medical achievement of the 20th century1. Studies suggest that in addition to making it feasible to treat infections, antibiotics also aid in performing a number of highly advanced medical procedures such as open-heart surgery, cancer treatment and organ transplantation2,3. Despite this great achievement, the misuse of antibiotics, among other factors, has led to the challenge of antimicrobial resistance, which is a burden4–6.
Microbes evolve to withstand antimicrobial treatments, rendering these drugs ineffective. This phenomenon, known as Antimicrobial Resistance (AMR), complicates infection management, potentially escalating disease transmission, morbidity and mortality rates7,8. Antimicrobial resistance is a major global public health threat, with the highest mortality rate in sub-Saharan Africa at 27.3 deaths per 100,000 people9,10. Severe infections caused by bacteria that are resistant to multiple drugs, especially carbapenem-resistant bacteria, are a threat to last-line antibiotics such as colistin. In the last few years, there has been a gradual increase in the prevalence of colistin resistance11. This highlights the need for more studies to understand the challenges of colistin resistance, especially in areas with high AMR-related mortality.
Colistin is an antibiotic used in both human and veterinary medicine. Colistin, classified as a polymyxin antibiotic, is one of only two polymyxins (along with polymyxin B) currently employed in human clinical practice12-14. It comprises two bactericidal compounds, polymyxin E1 and E2, which are pentacationic lipopeptides. These molecules target Gram-negative bacteria, compromising their outer membrane integrity and leading to cell death15. Healthcare providers primarily prescribe colistin to combat infections caused by bacteria exhibiting multidrug, extensive or pan-drug resistance patterns. It is not fully understood how colistin works, but it is thought to bind to lipopolysaccharides and phospholipids in the outer membrane of Gram-negative bacteria causing membrane disruption and consequently cell death16.
Studies show that pathogenic and potentially pathogenic bacteria, many of which may have antibiotic resistant genes, are constantly released into water environments with wastewater17,18. These genes get inserted into genetic mobile platforms such as plasmids and spread from water to soil17. Consequently, water is not only a means of spreading antibiotic-resistant organisms across human and animal populations, but it also introduces resistant genes into natural bacterial ecosystems. Non-pathogenic bacteria in such systems can serve as a repository of resistance genes and mobile platforms19. As a habitat for microbes, water can be the source of antibiotic resistance genes, a reservoir for genes that have already been acquired by human pathogens or a place where antibiotic resistance genes can be exchanged between different types of bacteria, indicating the important role water plays in the spread of antibiotic resistance17,19,20. However, despite the research in this area, it is still unclear under which circumstances water bacteria are important sources of novel mechanisms of antibiotic resistance.
As a crucial defence against multidrug-resistant Gram-negative pathogens, colistin plays a vital role in treating bacterial infections resistant to various antibiotics, including mcr-1 cephalosporins, quinolones, aminoglycosides and carbapenems21. However, the discovery of plasmid-mediated colistin resistance genes in carbapenem-resistant Enterobacteriaceae has raised significant concerns for both human and animal health, potentially compromising this last-resort antibiotic’s efficacy22. This emerging threat underscores the urgent need for antimicrobial stewardship and new treatment strategies.
Data on the prevalence of antimicrobial resistance is limited in Africa. In 2014, the World Health Organization (WHO) reported that antimicrobial resistance surveillance in Africa is particularly difficult due to a lack of reliable medical data and laboratory capacity23. Wrong healthcare practices, lax health systems and irresponsible behaviour by citizens all contribute to the challenges of antimicrobial resistance.
Social research is needed to understand the neighbourhood context and complexity of human behaviour that contribute to antimicrobial resistance. This study sought to assess the prevalence of colistin-resistant bacteria in water sources in the Wa Municipality of the Upper West Region of Ghana.
MATERIALS AND METHODS
Study design: This study followed a cross-sectional and experimental design pattern to assess the prevalence of colistin-resistant bacteria from three water sources; boreholes, wells and tap water in the selected communities within the Wa Municipality. The study was carried out from May, 2023 to July, 2023.
Sampling technique: The simple random sampling technique was used for communities with a large population while the stratified random sampling technique was used for communities with large populations.
Study site: Samples were obtained from the immediate environs of Dr. Hilla Limann Technical University (DHLTU), specifically Kpongu, Sawaba, Chokor and Bamahu, all within the Wa Municipality. The municipality is located in the Upper West Region of Ghana and shares boundaries to the North with Nadowli District, to the Southwest with Wa West District andthe East with Wa East District.
Study size and population: A total of 40 samples were collected from three different water sources; 16 samples from tap water, 16 samples from borehole water and 8 samples from well water spread across the selected communities.
Laboratory process: Samples, collected in sterile bottles, were promptly transported and analysed within 2 hrs. Water samples underwent filtration with a 0.22 μm syringe filter; the filter paper was placed in Brain Heart Infusion (BHI) broth and incubated at 35±°C for 18-24 hrs. The BHI cultures were sub-cultured onto MacConkey and Salmonella Shigella agar for general and selective isolation, respectively. Standard biochemical tests were used to identify isolates24 while Kirby-Bauer Disk diffusion and micro broth dilution techniques were used to assess colistin susceptibility. Colistin (10 μg) disks were placed on Mueller Hinton agar for colistin resistance screening according to Kirby-Bauer disk-diffusion method following standard laboratory protocols25. Antimicrobial susceptibility was interpreted using EUCAST standards. For microbroth dilution, BMD panels with colistin sulphate were prepared. Four Mueller-Hinton broths with varying colistin disks (0, 1, 2 and 4 μg/mL) were incubated and bacterial isolates were added26,27. The MIC values were visually read after 16-20 hrs at 35°C. Results were read after 18-24 hrs; growth was confirmed by turbidity. The test was valid if growth occurred in the control and no “skipped well” was observed.
Statistical analysis: Data was summarized, coded and merged in Microsoft Excel 2021, with the water samples as the unit of analysis, then imported into Statistical Package for Social Scientists (SPSS) version 28 set at a 95% confidence interval and a p-value of 0.05 as statistically significant for analysis of results using frequencies and proportions and presented in tables and charts.
RESULTS AND DISCUSSION
Community distribution and demographics of collected samples: The 40 water samples collected originated from 4 communities in the Wa Municipality, including Bamahu, Chokor, Kpongu and Sawaba. More samples were gathered from the higher population areas of Sawaba and Kpongu. The majority of samples (74.6%) came from urban settlements, compared to only 25.4% from rural settlements. This focus on communities near the regional capital is relevant, as higher population density and infrastructure access in urban areas impact water quality through direct wastewater discharge, pollutant runoff and overuse of antimicrobials28.
The 40 samples spanned three types of water sources used locally for drinking, washing and irrigation: Boreholes, taps and wells. Each source is contaminated by different microbes from human, animal, or environmental contact. Comparing results by source can reveal if certain points show higher resistance. Earlier studies revealed that borehole and well water sources contained higher bacteria contaminants compared to pipe-borne water29-31. Boreholes accessing vulnerable groundwater aquifers are also more contamination-prone than piped municipal supplies28.
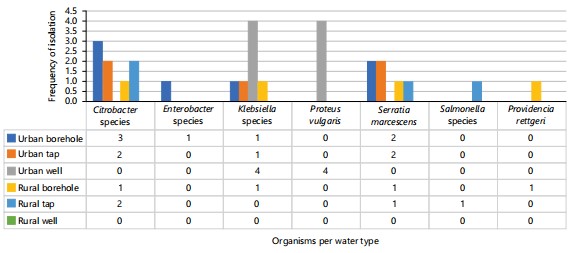
Prevalence and distribution of organisms isolated: Culture-based methods isolated a diversity of Gram-negative enteric organisms including Citrobacter species (28.5%), Klebsiella species (25%), Serratia marcescens (21.4%), Proteus vulgaris (14.3%), Enterobacter species (3.57%), Providencia rettgeri (3.57%) and Salmonella species (3.57%) from the water samples. Many clinically relevant drug-resistant genera were present, indicating widespread faecal contamination of water supplies from human and animal waste containing gut flora. Isolated organisms were distributed across the 4 communities without marked geographic predilections. However, some demographic associations emerged. Klebsiella species predominated among urban samples, while Citrobacter species dominated in rural settings (Fig. 1).
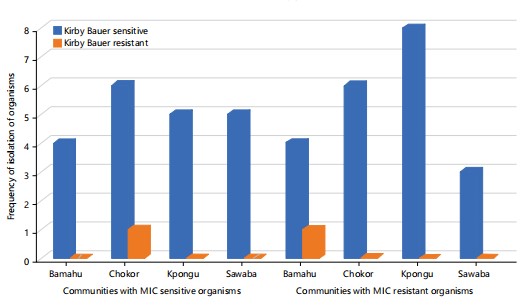
Phenotypic resistance testing methods and findings: Phenotypic colistin resistance was evaluated using both Kirby Bauer disk diffusion (KBDD) and broth microdilution minimum inhibitory concentration (MIC) quantification. The KBDD method indicated only 4.7% of isolates as resistant, while the MIC testing found 44.8% of isolates as resistant. This discrepancy highlights the superior sensitivity of MIC for detecting colistin resistance described in some earlier studies32,33. However, substantial resistance identified by both methods among community water samples without recent clinical antimicrobial pressure is alarming.
The MIC testing revealed resistant organisms occurring across all 4 communities (Fig. 2). In contrast, KBDD detected limited resistance, with single isolates in Chokor and Bamahu. Widespread resistance by MIC suggests colistin-resistant bacteria are prevalent through water sources in the Wa Municipality due to common antimicrobial contamination pressures and resistance gene exchange between organisms19. However, KBDD may still provide useful preliminary resistance screening where resources are constrained.
Statistical analysis also found a significant positive correlation between urban settlements and resistance detected via MIC testing (p = 0.049), indicating human population activities as likely resistance drivers. No other clear correlations between community, source or method were identified (Table 1).
|
|
| Table 1: | Correlation between community, settlement, water type, disk diffusion inhibition zone and MIC status | |||
| Community | Settlement | Water type | Disc diffusion zone | Mic status | ||
| Community | Pearson Correlation | 1 | 0.165 | 0.122 | 0.017 | -0.172 |
| Sig. (2-tailed) | 0.211 | 0.358 | 0.913 | 0.196 | ||
| N | 59 | 59 | 59 | 43 | 58 | |
| Settlement | Pearson Correlation | 0.165 | 1 | -0.268* | 0.243 | 0.259* |
| Sig. (2-tailed) | 0.211 | 0.04 | 0.117 | 0.049 | ||
| N | 59 | 59 | 59 | 43 | 58 | |
| Water type | Pearson Correlation | 0.122 | -0.268* | 1 | -0.209 | -0.033 |
| Sig. (2-tailed) | 0.358 | 0.04 | 0.178 | 0.805 | ||
| N | 59 | 59 | 59 | 43 | 58 | |
| Disc diffusion | Pearson Correlation | 0.017 | 0.243 | -0.209 | 1 | 0.103 |
| zone | Sig. (2-tailed) | 0.913 | 0.117 | 0.178 | 0.511 | |
| N | 43 | 43 | 43 | 43 | 43 | |
| Mic status | Pearson Correlation | -0.172 | 0.259* | -0.033 | 0.103 | 1 |
| Sig. (2-tailed) | 0.196 | 0.049 | 0.805 | 0.511 | ||
| N | 58 | 58 | 58 | 43 | 58 | |
| *Correlation is significant at the 0.05 level (2-tailed) | ||||||
Microbiological findings by source and location: Among urban sam ples, numerically more resistant organisms originated from borehole water than the others (Fig. 1). This trend aligned with prior studies finding groundwater supplies more vulnerable to contaminant infiltration28. Within rural areas, the diversity of flora appeared lower but more organisms were isolated from boreholes than taps or wells (Fig. 1).
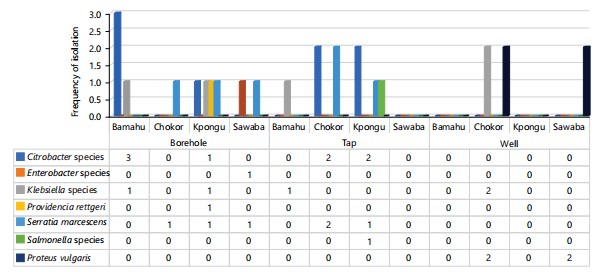
Breaking down results by community, variability emerged in the organism profiles and resistance levels across different sources:
| • | Boreholes (Fig. 3): More Klebsiella species in Kpongu/Chokor; more Serratia marcescens in Bamahu/Sawaba. This indicates potential small-scale differences in contaminant sources and selective pressures within locations | |
| • | Taps (Fig. 3): Salmonella species uniquely found in Chokor’s piped groundwater indicates alarming fecal contamination which is possibly due to breaches in distribution system integrity or contaminated aquifers possible | |
| • | Wells (Fig. 3): Numerically more Klebsiella species and Proteus vulgaris weredetected in Chokor/Kpongu wells. This suggests possible groundwater contamination by surface runoff, aquifer leakage, or direct fouling. Well depth and construction quality are risks |
Together, these points to urbanization and reliance on vulnerable groundwater as likely increasing exposure to resistant bacteria through drinking, domestic and agricultural water use and resistance mechanisms continue to spread through all water sources.
Susceptibility profiles of isolated organisms: Analyzing susceptibility against source, location and organism variables also revealed informative trends: More diverse resistant organisms were found in urban compared to rural areas (8 types against 5 types) (Table 2). The only rural resistance was detected in Klebsiella species which showed intermediate resistance to colistin. Urban locations haboured additional resistant organisms (Klebsiella, Proteus, Serratia and Citrobacter species) primarily in boreholes and wells; indicating human impacts on resistance. By MIC testing, resistant phenotypes were detected in all 4 communities. Highest resistance prevalence was in Kpongu (Citrobacter and Serratia species) and Chokor (Klebsiella species and Proteus species). Lowest was in Bamahu (Klebsiella species). This reflects spatial heterogeneity between closely situated locations.
The MIC also identified resistant organisms (Klebsiella species, Citrobacter species and Salmonella species) in rural areas, whereas KBDD found none (Table 2). This underscores MIC’s superior sensitivity. Resistance spanned all water sources (boreholes, taps, wells) and organisms (Klebsiella species, Proteus species and Serratia species).
This study revealed high levels of colistin-resistant Gram-negative bacteria contaminating drinking water sources in the Wa Municipality of Ghana. Nearly half of all isolates tested exhibited phenotypic colistin-resistance based on the sensitive broth microdilution minimum inhibitory concentration testing method. Resistant organisms were diverse, spanning Klebsiella, Citrobacter, Salmonella, Serratia species and other clinically relevant genera that are common gut flora.
Statistical analysis identified a significant association between urban settlements and colistin-resistance. Numerically more resistant organisms originated from boreholes than the other sources, especially in urban areas, reflecting vulnerabilities in accessed groundwater. There was also evidence of small-scale spatial heterogeneity in contamination and resistance profiles between closely situated communities.
Overall, findings likely reflect widespread faecal pollution from human and animal waste introducing gut flora and resistance genes into water systems. Unregulated antimicrobial use and poor waste management appear to account for resistance. The high prevalence of colistin-resistant enteric bacteria in drinking water presents a major public health risk in the Wa Municipality.
Improving sanitation infrastructure, water treatment and hygiene practices is critical to reducing faecal pollution from human and animal waste introducing resistant gut flora into water systems. Regulating antimicrobial use through stewardship programs and prescriber/consumer education is also essential to curb selection pressure driving resistance. Ongoing surveillance utilizing sensitive MIC testing should monitor resistance trends and identify vulnerable points in water systems requiring priority remediation, whether boreholes tapping contaminated aquifers or aging pipe distribution networks. A coordinated “One Health” approach across public health, veterinary, environmental and local governance sectors is necessary to control the selection and spread of antimicrobial resistance in this region.
|
| Table 2: | Cross-tabulation of organisms isolated, resistance testing methods and settlement type | |||
| Disc diffusion status | MIC status | ||||
| Settlement | Organism isolated | Sensitive | Resistance | Sensitive | Resistance |
| Urban | Citrobacter species | 5 | 0 | 2 | 3 |
| Enterobacter species | 1 | 0 | 0 | 1 | |
| Klebsiella species | 3 | 1 | 2 | 4 | |
| Proteus vulgaris | 2 | 0 | 3 | 1 | |
| Serratia marcescens | 4 | 0 | 2 | 2 | |
| Rural | Citrobacter species | 3 | 0 | 1 | 2 |
| Klebsiella species | 1 | 0 | 0 | 1 | |
| Providencia rettgeri | 1 | 0 | 1 | 0 | |
| Serratia marcescens | 2 | 0 | 1 | 1 | |
| Salmonella species | 3 | 0 | 0 | 1 | |
CONCLUSION
This study reveals high levels of colistin-resistant bacteria in Wa Municipality’s drinking water sources, posing a significant public health risk. Urban areas and boreholes showed higher resistance rates, indicating human activity impacts. Addressing this issue requires improving sanitation, water treatment, hygiene practices and antimicrobial stewardship. Immediate action and further research are crucial to combat resistance spread, safeguard community health and preserve antibiotic efficacy for future generations.
SIGNIFICANCE STATEMENT
This study addresses the critical gap in understanding antimicrobial resistance in water sources in Ghana, focusing on colistin resistance. The research is significant as it reveals high levels of colistin-resistant Gram-negative bacteria in drinking water sources in the Wa Municipality, with 44.8% of isolates showing resistance. The findings highlight the potential public health risk and the need for improved water management and antimicrobial stewardship. The study uniquely demonstrates the superiority of MIC testing over disk diffusion for detecting colistin resistance and identifies a significant association between urban settlements and resistance. These results contribute valuable data to inform public health strategies in Ghana and similar settings, emphasizing the importance of a One Health approach in combating antimicrobial resistance.
ACKNOWLEDGMENT
The authors of this study are grateful to the individuals who granted us access to their homes for sampling.
REFERENCES
- Katz, L. and R.H. Baltz, 2016. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol., 43: 155-176.
- Bromberg, J.S., J.R. Scalea and E.F. Mongodin, 2019. De-bugging the system: Could antibiotics improve liver transplant outcomes? J. Clin. Invest., 129: 3054-3057.
- Kappeler, R., M. Gillham and N.M. Brown, 2012. Antibiotic prophylaxis for cardiac surgery. J. Antimicrob. Chemother., 67: 521-522.
- Moussa, A.A. and B. Garba, 2022. How misuse of antimicrobial agents is exacerbating the challenges facing Somalia's public health. Afr. J. Infect. Dis., 16: 26-32.
- Serwecińska, L., 2020. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water, 12.
- Bianco, A., F. Licata, R. Zucco, R. Papadopoli and M. Pavia, 2020. Knowledge and practices regarding antibiotics use: Findings from a cross-sectional survey among Italian adults. E Med. Public Health, 2020: 129-138.
- Kollef, M.H., 2008. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: Getting it right up front. Clin. Infect. Dis., 47: S3-S13.
- Paul, M., V. Shani, E. Muchtar, G. Kariv, E. Robenshtok and L. Leibovici, 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother., 54: 4851-4863.
- ARC, 2022. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet, 399: 629-655.
- Prestinaci, F., P. Pezzotti and A. Pantosti, 2015. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Global Health, 109: 309-318.
- El-Mahallawy, H.A., M. El Swify, A. Abdul Hak and M.M. Zafer, 2022. Increasing trends of colistin resistance in patients at high-risk of carbapenem-resistant Enterobacteriaceae. Ann. Med., 54: 2748-2756.
- Dijkmans, A.C., E.B. Wilms, I.M.C. Kamerling, W. Birkhoff and N.V. Ortiz-Zacarías et al., 2015. Colistin: Revival of an old polymyxin antibiotic. Ther. Drug Monit., 37: 419-427.
- Son, S.J., R. Huang, C.J. Squire and I.K.H. Leung, 2019. MCR-1: A promising target for structure-based design of inhibitors to tackle polymyxin resistance. Drug Discovery Today, 24: 206-216.
- Falagas, M.E., S.K. Kasiakou and L.D. Saravolatz, 2005. Colistin: The revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin. Infect. Dis., 40: 1333-1341.
- Tambadou, F., T. Caradec, A.L. Gagez, A. Bonnet and V. Sopéna et al., 2015. Characterization of the colistin (polymyxin E1 and E2) biosynthetic gene cluster. Arch. Microbiol., 197: 521-532.
- Kaye, K.S., J.M. Pogue, T.B. Tran, R.L. Nation and J. Li, 2016. Agents of last resort: Polymyxin resistance. Infect. Dis. Clin. North Am., 30: 391-414.
- Gulkowska, A., H.W. Leung, M.K. So, S. Taniyasu and N. Yamashita et al., 2008. Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water Res., 42: 395-403.
- Karkman, A., T.T. Do, F. Walsh and M.P.J. Virta, 2018. Antibiotic-resistance genes in waste water. Trends Microbiol., 26: 220-228.
- Baquero, F., J.L. Martínez and R. Cantón, 2008. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol., 19: 260-265.
- Rizzo, L., C. Manaia, C. Merlin, T. Schwartz and C. Dagot et al., 2013. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ., 447: 345-360.
- Zavacki, A.P., L.Z. Goldani, J. Li and R.L. Nation, 2007. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother., 60: 1206-1215.
- Liu, Y.Y., Y. Wang, T.R. Walsh, L.X. Yi and R. Zhang et al., 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis., 16: 161-168.
- Yewale, V.N., 2014. Antimicrobial resistance-A ticking bomb! Indian Pediatr., 51: 171-172.
- Mridha, D., M.N. Uddin, Badrul Alam, A.H.M.T. Akhter and S.K.S. Islam et al., 2020. Identification and characterization of Salmonella spp. from samples of broiler farms in selected districts of Bangladesh. Vet. World, 13: 275-283.
- Satlin, M.J., J.S. Lewis, M.P. Weinstein, J. Patel and R.M. Humphries et al., 2020. Clinical and laboratory standards institute and european committee on antimicrobial susceptibility testing position statements on polymyxin B and colistin clinical breakpoints. Clin. Infect. Dis., 71: e523-e529.
- Kahlmeter, G., D.F.J. Brown, F.W. Goldstein, A.P. MacGowan and J.W. Mouton et al., 2006. European committee on antimicrobial susceptibility testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin. Microbiol. Infect., 12: 501-503.
- Yusuf, E., M. van Westreenen, W. Goessens and P. Croughs, 2020. The accuracy of four commercial broth microdilution tests in the determination of the minimum inhibitory concentration of colistin. Ann. Clin. Microbiol. Antimicrob., 19.
- Vaz-Moreira, I., O.C. Nunes and C.M. Manaia, 2014. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev., 38: 761-778.
- Dekker, D.M., R. Krumkamp, N. Sarpong, H. Frickmann and K.G. Boahen et al., 2015. Drinking water from dug wells in rural Ghana-Salmonella contamination, environmental factors, and genotypes. Int. J. Environ. Res. Public Health, 12.
- Lutterodt, G., J. van de Vossenberg, Y. Hoiting, A.K. Kamara, S. Oduro-Kwarteng and J.W.A. Foppen, 2018. Microbial groundwater quality status of hand-dug wells and boreholes in the Dodowa Area of Ghana. Int. J. Environ. Res. Public Health, 15.
- Elijah, E.U., 2023. Bacteriological assessment of pipe-borne, borehole, and well water sources available to students in Nasarawa State University Keffi, Nasarawa State, Nigeria. Sustinere: J. Environ. Sustainability, 7: 112-121.
- Yadav, S.K., R. Sujatha, S. Kumar and A. Nashra, 2023. Evaluation the phenotypic assays for the detection of colistin resistance in Klebsiella pneumoniae isolates from Critical Care Units in Tertiary Care Hospital, Kanpur. Biochem. Cell. Arch., 23: 1125-1131.
- Ghandour, A., S. Sror, M. Sabet and R. Rashwan, 2021. Detection of colistin resistant E. coli in children at Pediatric hospital of Assiut University, using phenotypic and genotypic methods. Microbes Infect. Dis., 2: 748-759.
How to Cite this paper?
APA-7 Style
Osisiogu,
E.U., Kombat,
M., Alhassan,
B., Ayishetu,
M., Kinawie,
J., Amemo,
R.E., Ahadzie,
S.Y., Asoandzie,
D.N., Bawa,
F.K., Agyei,
S.A. (2024). Prevalence of Colistin-Resistant Bacteria in Water Sources in Wa, Upper West Region of Ghana. Science International, 12(1), 21-29. https://doi.org/10.17311/sciintl.2024.21.29
ACS Style
Osisiogu,
E.U.; Kombat,
M.; Alhassan,
B.; Ayishetu,
M.; Kinawie,
J.; Amemo,
R.E.; Ahadzie,
S.Y.; Asoandzie,
D.N.; Bawa,
F.K.; Agyei,
S.A. Prevalence of Colistin-Resistant Bacteria in Water Sources in Wa, Upper West Region of Ghana. Sci. Int 2024, 12, 21-29. https://doi.org/10.17311/sciintl.2024.21.29
AMA Style
Osisiogu
EU, Kombat
M, Alhassan
B, Ayishetu
M, Kinawie
J, Amemo
RE, Ahadzie
SY, Asoandzie
DN, Bawa
FK, Agyei
SA. Prevalence of Colistin-Resistant Bacteria in Water Sources in Wa, Upper West Region of Ghana. Science International. 2024; 12(1): 21-29. https://doi.org/10.17311/sciintl.2024.21.29
Chicago/Turabian Style
Osisiogu, Emmanuel, Udochukwu, Mathew Kombat, Barikisu Alhassan, Mahmud Ayishetu, John Kinawie, Raphael Eyram Amemo, Senam Yawa Nunamey Ahadzie, Doreen Ntsiakoa Asoandzie, Flavia Kaduni Bawa, and Samuel Adu Agyei.
2024. "Prevalence of Colistin-Resistant Bacteria in Water Sources in Wa, Upper West Region of Ghana" Science International 12, no. 1: 21-29. https://doi.org/10.17311/sciintl.2024.21.29

This work is licensed under a Creative Commons Attribution 4.0 International License.



